How to correctly distinguish LPC I
I’m still wondering how it is possible that some LPC systems need a batch surface area, others don’t, some LPC systems produce soot and others don’t, and how it is possible that some types of LPC throw away a lot of graphite heating elements every year, and other systems claim to last years without maintenance.
Is there a difference between them? Yes, it is, but it really is infinitely large. I tried to find a comparison of the AllCarb system from Fours BMI, SimVac from SecoWarwick, Ipsen Avac, ECM InfraCarb and IHI, offered with the name “The continuous vacuum carburizing”.
First, let’s go through the reasons why soot and tar fumes are formed in the furnace. I came across the basic hypothesis in the material of the ULVAC company, which produces mass spectrometers.
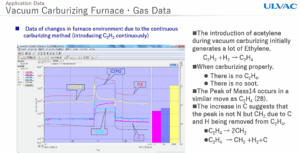
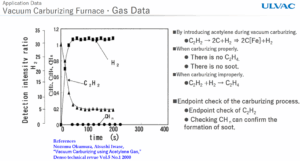
Fig. 1 a 2 – Measurement of the atmospheric spectrum with a mass spectrometer from ULVAC
But how do you explain that one way and the other time differently? Normally, C2H2 decomposes catalytically as mentioned above. In contact with the surface of steel parts. However, if there is an excess of it and it does not only decompose catalytically on the surface of the metal, but in the volume of the furnace — carbon soot is formed, which settles on parts and walls. This is reflected in the mass spectrum of the atmosphere at m/e = 14 and 28. The peak m/e = 14 is not nitrogen but CH2, and is a manifestation of the reaction C2H4 → 2 CH2. This means that at the same time the basic peak for acetylene m/e = 28 decreases. The next decomposition is then C2H4 (28) → CH2(14) + H2(2) + C(12), and the carbon at the end is the excess carbon.
Therefore, if C₂H₂ is consumed quickly and C₂H₄ remains low, the carburization takes place correctly (reaction on the surface, no soot).
If C₂H₂ remains high or C₂H₄ starts to grow, pyrolysis occurs. This leads to the formation of soot, loss of efficiency, the need to adjust parameters (pressure, flow, boost duration).
The ideal reaction takes place heterogeneously on the surface of the steel, where the atomic carbon dissolves into the surface.
- The result is: a decrease in C₂H₂, a slight increase in H₂, no C₂H₄.
- The surface acts as a catalyst and at the same time as a “cesspool” for carbon. Simply put, everything is consumed in steel.
If the conditions change in such a way that C₂H₂ does not decompose catalytically on the metal, but begins to react homogeneously in the gas, reactions such as:
2 C2H2 → C4H4 → C2H4 + 2 C (soot)
or to put it simply:
C4H4 → C2H4 + 2 C
Thus, the resulting C₂H₄ (ethylene) is the product of uncontrolled C₂H₂ pyrolysis. What invoices affect this?
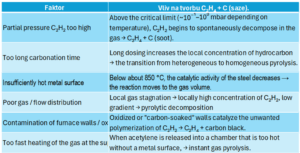
Fig. 3 a 4 – Factors affecting sooting
The indication of the transition between optimal and non-optimal carburizing is as follows:
Optimal carburizing:
- C₂H₂ drops sharply after injection into the furnace
- C₂H₄ ≈ 0,
- H₂ rises slightly.
Risky carburizing with sooting:
- C₂H₂ remains high,
- C₂H₄ starts to rise (clear warning sign),
- H₂ can rise sharply (decomposition in volume).
Ethylene (C₂H₄) in a vacuum chamber is formed when acetylene is exceeded — that is, when C₂H₂ decomposes in a volume of gas instead of on the surface of steel. This occurs at high partial pressure, inappropriate temperature, long boost or poor flow. A properly set LPC process (boost short, pressure ~0.5–2 mbar, temperature > 900 °C, good flow) leads to only C₂H₂ → H₂ + C(Fe) without C₂H₄ formation.
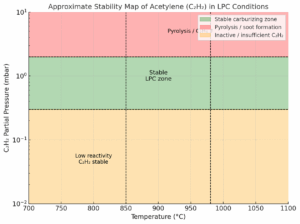
Stable area for LPC
- Temperature: ~900–980 °C
- Partial pressure C₂H₂: ≈3–2 mbar
- H₂/C₂H₂ ratio: > 2 (controlled inert or carrier gas supply)
In this area, the catalytic decomposition of acetylene takes place directly on the surface of the steel; no C₂H₄, minimum CH₄.
Unstable area
- C₂H₂ pressure > 5 mbar or insufficient flow → spontaneous polymerization of C₂H₂ → C₂H₄, C₄H₄, carbon black
- Low temperature (< 850 °C) → metal inactive, decomposition moves to a volume of → C₂H₄ + C.
Converted into a graph, it looks like this:
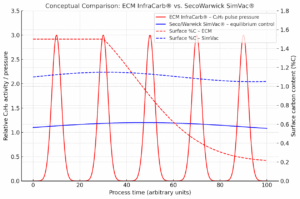
Fig. 5 a 6 – Stability band chart for LPC and comparison of the behavior of InfraCarb from ECM Technologies and SimVac from SecoWarwick.
- 🟢 Green area – stable carburizing (surface decomposition, no C₂H₄).
- 🟠 Orange – low temperature/pressure → insufficient activation, C₂H₂ hardly decomposes.
- 🔴 Red – too high pressure or poor flow → pyrolysis, formation of C₂H₄ and soot.
And what is the difference between, for example, the ECM InfraCarba SimVac from SecoWarwick?
🔴 ECM InfraCarb® – short, strong pulses of acetylene (high C₂H₂ activity) that temporarily oversaturate the surface above the Acm line → short-term Fe₃C is formed, from which carbon diffuses into austenite.
🔵 Seco/Warwick SimVac® – smooth, low-intensity control of the atmosphere so that the surface remains at equilibrium “carbon potential” (e.g. 1.1 %C) — without switching to Fe₃C.
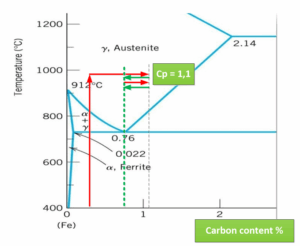
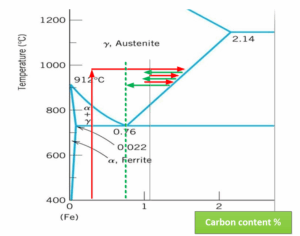
The problem is that in the first case they don’t need to know the area, the equilibrium is established automatically on the line Acm, with SimVac I need to know the size of the area to establish the equilibrium on a certain saturation potential. But if I miss, I have a good chance of switching to C2H4 mode with all the consequences. The principle is therefore similar to that of Cp control in multi-purpose furnaces.
Fig. 7 a 8 – Graphical presentation of the difference between SimVac (left) and InfraCarb (right)
Of course, there are other factors that contribute to the formation of soot in the combustion chamber. We will talk about this in the next part.
Jiří Stanislav
November 1, 2025